Effect of Stepped PK Constant Flow Formation Process on SEI and Electrochemical Performance of Battery
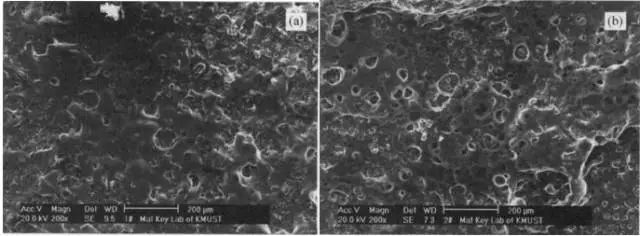
On the one hand, the formation of the SEI film consumes a part of lithium ions, which makes the irreversible capacity of the first charge and discharge increase, and reduces the charge and discharge efficiency of the electrode material; on the other hand, the SEI film has an organic solvent insolubility and can stably exist in the organic electrolyte solution. Can improve the cycle life of lithium batteries. The chemical conversion process has an important influence on the formation of the SEI film of the lithium battery, and also directly affects the performance of the battery. Generally, the formation potential of the SEI film is in the range of 0.6V-0.8V, so the initial current in the formation of the SEI film tends to be kept at an extremely small state to ensure the formation of the SEI film is more dense, which is advantageous for the improvement of the cycle life. Although the traditional small current pre-charging method contributes to the formation of a stable SEI film, long-term small current charging causes an increase in the impedance of the formed SEI film, thereby affecting the cycle, rate performance, etc. of the battery; Affects the formation of the SEI film of the battery, because the formation of the lithium ion battery is a process of first activation. As the charging progresses, the internal voltage of the battery rises with the generation of gas, and once the gas production rate is higher than that of the injection hole At the rate, the gas will accumulate between the diaphragms inside the battery, which will affect the formation of the SEI film on the surface of the negative electrode, so it is necessary to select a suitable current and formation time. At present, the chemical conversion process is mainly divided into two types, a multi-step stepwise chemical conversion process and a constant flow chemical conversion process. Which chemical conversion process is more suitable? Group A multi-step formation process: Charging (0.05CC/4h→0.1CC/2h→0.2CC/1h→0.4CC CV/4.2V→0.02Ccut)→stationary 0.5h→discharge (0.5C to cutoff voltage)→stationary 0.5h, cycle three times and then 0.2 C/2h, charging to 4.0V Group B uses a constant flow formation process: Charging (0.2C/2.5h)→Stilling 12h→Discharging (0.2C cut-off voltage)→Stilling 0.5h→Charging (0.2C/4.2V, 0.02C cut-off)→Stilling 0.5h→Discharging (0.2C cut-off voltage) →Still 0.5h→Charging (0.2C/4.0V) As you can see, Group A charging uses a small current and slow charge, gradually increasing the current mode, while reducing the charging time. Group B uses 0.2C charge/discharge current, and there are not many parameters to change. So what are the differences between the two chemical formation processes for the SEI and electrochemical performance of the battery? First, SEI film By performing SEM analysis on the negative electrode surfaces of the two groups of cells, it was found that the surface of the electrode was covered by the SEI film, but it was not possible to see the difference in thickness or coverage area between the two. Second, electrochemical performance By analyzing the basic electrochemical performance of the two groups of batteries, it can be concluded that the positive electrode material capacity of the lithium battery adopting the stepwise formation is 3 mAh/g higher than that of the constant current formation process, and the charge and discharge efficiency of the whole battery is higher. After a 50-week cycle, the constant current conversion rate is slower than the stepwise formation process. In the first effect, the constant current type is lower than the step type, but the second cycle is higher than the step type, which also explains that the reversible reaction degree of the battery formed by the constant current method is higher than that of the stepwise formation process, that is, Less irreversible capacity loss. Third, SEI component analysis By analyzing the SEI of the two sets of batteries, the following conclusions can be drawn: 1. After the stepwise formation, the lithium ion content of the surface of the negative electrode of the lithium ion battery is higher than that of the constant current type. This is because a variety of lithium-containing compounds are formed under different current densities, resulting in excessive lithium content. 2. XPS results show that Li2CO3 or LiCO2-R is present in the SEI membrane of both groups of cells. 3. The SEI thickness formed by the two formation processes is greater than 3 nm. Through the above two groups of different chemical process battery analysis, it can be concluded that different current magnitudes and times have different effects on the performance of the battery. The composition and properties of the SEI film are also different, which will inevitably affect the performance of the battery. In actual production, it is necessary to adjust the current magnitude and time according to the battery system and capacity, and it is not reasonable to say that a certain method is unreasonable, but no matter which method is adopted, it is beneficial to the generation of SEI and the method for improving its stability. It is inevitable that this article is only for communication and learning. Welcome to leave a message. Stretch Bar Display,Stretched Bar Lcd Display,Digital Advertising Screen,Stretch Bar Lcd Display APIO ELECTRONIC CO.,LTD , https://www.displayapio.com
After the lithium battery is filled, during the first charge and discharge process, the electrode material and the electrolyte will electrochemically react at the solid-liquid interface to form a solid electrolyte interface film (SEI film) covering the surface of the electrode material, SEI The quality of the membrane directly determines the cycle performance of the battery. SEI is composed of Li2O, LiF, LiCl, Li2CO3, LiCO2-R, alkoxide and non-conductive polymer. It is a multi-layer structure, one side close to the electrolyte is porous, and one side close to the electrode is dense.